Dermal Filler Do’s and Don’ts for Wrinkles, Lips and More
Impression
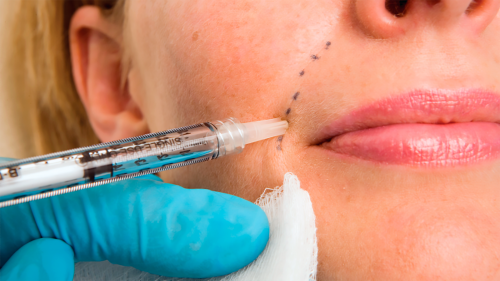
Individuals are trying to find remedies to clean smile traces and crow’s ft and plump up their lips, cheeks, and palms.
Injecting dermal fillers into the confront and palms can enhance the physical appearance of facial traces and quantity loss induced by age or sure medical disorders. In reports of dermal fillers permitted by the U.S. Foodstuff and Drug Administration, individuals generally report they are content with their procedure final results.
On the other hand, dermal fillers are not for all people. Dermal fillers could not be ideal for individuals with sure disorders, this kind of as bleeding conditions or some allergies. If your wellness treatment supplier confirms that dermal fillers are an alternative for you, know that all medical items have rewards and risks. The Fda advises you work with a accredited wellness treatment supplier who is experienced in injecting dermal fillers, well-informed about fillers, anatomy, taking care of troubles, and most importantly, tells you about the risks and rewards just before getting procedure.
What are dermal fillers?
Dermal fillers are gel-like substances injected under the pores and skin. Dermal fillers are intended to create a smoother or fuller physical appearance, or both equally.
The Fda regulates dermal fillers as medical devices. As described in scientific trials, the consequences of most Fda-permitted dermal fillers are momentary simply because they are designed from elements that the human body at some point breaks down and absorbs. The injection procedure could have to be repeated to keep the sought after effect.
Kinds of dermal fillers
Momentary fillers incorporate the adhering to elements:
- Hyaluronic acid, a sugar that is the natural way identified in the human body
- Calcium hydroxylapatite, a mineral and a important ingredient of bone
- Poly-L-lactic acid (PLLA), a biodegradable, artificial material
There’s only a single Fda-permitted dermal filler that is not absorbed by the human body. It is designed with polymethylmethacrylate (PMMA) beads suspended in a solution that contains bovine (cow) collagen. PMMA beads are little round, clean, plastic beads.
Fda-permitted makes use of of dermal fillers
Dermal fillers are permitted for certain makes use of in individuals aged 22 and older. Those people makes use of incorporate:
- Correcting average-to-critical facial wrinkles and pores and skin folds
- Raising fullness of lips, cheeks, chin, under-eye hollows, jawline, and again of the hand
- Restoring facial fats loss in individuals with human immunodeficiency virus (HIV)
- Correcting pimples scars on the cheek
Fda warnings about unapproved fillers
- The Fda has not permitted injectable silicone or any injectable fillers for human body contouring or enhancement. The Fda has warned versus getting filler injected into the breasts, buttocks, or spaces between the muscle tissue. Working with injectable filler for huge-scale human body contouring or human body enhancement can lead to major injuries, like extended-term agony, infection, lasting scarring or disfigurement, and even loss of life.
- The Fda has not permitted needle-free of charge devices for the injection of dermal fillers and warns versus applying them to inject hyaluronic acid or other lip and facial fillers. The injectors use superior pressure and do not present plenty of manage around wherever filler will be positioned. Serious accidents and in some scenarios, lasting harm to the pores and skin, lips or eyes have transpired.
- The Fda also warns versus purchasing or applying lip or facial fillers that are bought specifically to the general public. They are not Fda permitted and could be contaminated with chemical substances and infectious organisms. The only Fda-permitted dermal fillers are provided by a prescription for injection by a accredited wellness treatment skilled applying a syringe with a needle or a cannula (a tiny flexible tubing with a blunt tip that is inserted under the pores and skin).
Threats of Fda-permitted fillers
As with any medical procedure, there are risks concerned with the use of dermal fillers. Most side consequences described in scientific trials and publish-industry surveillance take place shortly after injection and go absent in just a several months. In some scenarios, side consequences could emerge months, months, or a long time later.
Frequent risks incorporate:
- Bruising
- Redness
- Inflammation
- Discomfort
- Tenderness
- Itching
- Rash
- Difficulty in executing functions (only noticed when injected into the again of the hand)
Individuals need to be tested for allergies just before getting dermal fillers designed with sure elements, in particular elements derived from animals, this kind of as collagen.
Unintended injection into blood vessels
The most major danger linked with dermal fillers is accidental injection into a blood vessel. Filler that enters a blood vessel can induce pores and skin necrosis (loss of life of tissue), stroke, or blindness. Even though the odds of this happening are low, if it does come about, the ensuing troubles can be major and could be lasting.
Taking away Dermal Fillers
If you want to have fillers taken out or lessened simply because of side consequences, you could need to have added techniques to cut down the filler or surgical procedures to get rid of it. These techniques carry their possess risks. Be conscious that it could be difficult or unattainable to get rid of some filler elements.
six Tips for Buyers About Injectable Dermal Fillers
- Do work with a accredited wellness treatment supplier who has experience in the fields of dermatology or plastic surgical procedures and is educated to inject dermal fillers. The supplier need to use properly labeled, sealed vials or pre-crammed syringes of Fda-permitted filler.
- Do request and study the client labeling data on Fda-permitted injectable dermal fillers from your accredited wellness treatment supplier.
- Do know the style of solution to be injected and the feasible risks. Know wherever each and every solution you will be getting is to be injected. Talk to your accredited wellness treatment supplier if you have any thoughts.
- Do not buy dermal fillers that are bought specifically to the general public. They could be fake, contaminated, or not permitted for use in the U.S. Fda-permitted dermal fillers are indicated for prescription use only.
- Do not inject by yourself with dermal fillers or with needle-free of charge injection “pens.”
- Do not get any style of filler or liquid silicone injected for human body contouring.
Dermal Fillers and Botulinum Toxin Items
The Fda also has permitted botulinum toxin items this kind of as Botox, Dysport, Xeomin and Jeuveau to address facial wrinkles. These items are not dermal fillers. They are injectable medicines that work by keeping muscle tissue from tightening, so the wrinkles really don’t exhibit as considerably. The risk-free use of dermal fillers in blend with Botox and other remedies has not been evaluated in scientific reports.
Despite the fact that botulinum toxin items are derived from the exact germs that induce botulism, the amounts utilised for beauty applications are purified and many orders of magnitude smaller sized.
The Fda has permitted these injectable medicines for the momentary advancement in the physical appearance of a single, or probably various varieties of facial traces, like frown traces, brow traces, and crow’s ft.
Facet consequences described in scientific trials incorporate facial weak point, eyelid drooping, and brow drooping. Other adverse events incorporated localized agony, inflammation, reddening, and bruising at the injection web-site. In scarce scenarios, injections have resulted in double vision, dry eyes, or problem swallowing or respiration. The injection of botulinum toxin items for beauty applications is not advisable for use whilst pregnant or lactating.
More Data
If you have experienced a dilemma with a dermal filler solution or other solution regulated by the Fda, you can voluntarily report it to MedWatch, the Fda security data and adverse event reporting software.
