Infant Formula Recall: What to Know
Image

Español
If you use powdered toddler formula, be aware specified Similac, Alimentum and EleCare goods have been recalled and ought to not be used.
The U.S. Food items and Drug Administration (Fda) is investigating buyer issues of bacterial bacterial infections in 5 infants who eaten powdered infant method generated in Abbott Nutrition’s facility in Sturgis, Michigan. All five infants had to be hospitalized and the bacterial an infection could have contributed to death in two people.
Simply because toddler formulation is the only source of diet for lots of newborns and infants, the Fda understands and shares the problems moms and dads and caregivers may well have.
Here’s info to support you as we keep on our investigation.
What powdered toddler formula products and solutions have been recalled?
Abbott Nutrition has recalled sure powdered infant method items developed at its Sturgis, Michigan facility. Products and solutions from that facility can be found throughout the U.S. and some had been exported to other nations around the world. Here’s how you can inform if you have any of those items.
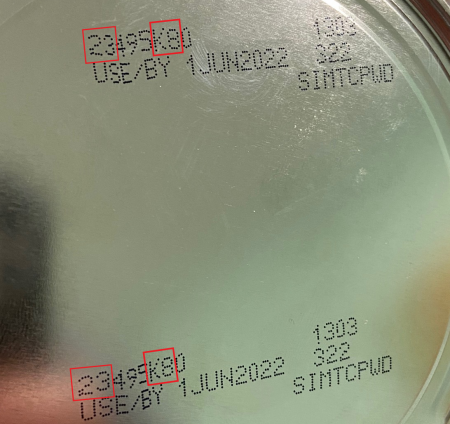
The Fda endorses people look at the ton code, a multidigit range on the base of a container of Similac, Alimentum and EleCare powdered toddler formulation and do not use if:
- the 1st two digits of the code are 22 through 37 and
- the code on the container contains K8, SH or Z2 and
- the expiration day is 4-1-2022 (APR 2022) or later.
In addition to goods explained higher than, Abbott Nutrition has recalled Similac PM 60/40 with a great deal code of 27032K80 (can) / 27032K800 (situation).
You can also enter your merchandise whole lot code on the company’s internet site to verify if it is aspect of the recall. Remember to see the photos down below for a nearer seem at the determining information.
Powdered Abbott merchandise that really don’t have the code and expiration noted earlier mentioned are not bundled in the recall. Liquid method items are not issue to the remember. At this time, Similac PM 60/40 with ton code 27032K80 (can) / 27032K800 (situation) are the only type and lots of this specialty components being recalled.
What infections have been described and what indications should really I search for?
Four circumstances require Cronobacter sakazakii, and a person involves Salmonella Newport an infection.
- Cronobacter microbes can bring about significant, existence-threatening bacterial infections (sepsis) or meningitis (an inflammation of the membranes that guard the brain and spine). Cronobacter bacterial infections are uncommon but are in particular substantial danger for newborns.
- Salmonella are a group of germs that can result in gastrointestinal health issues and fever referred to as salmonellosis.
- Symptoms related to Cronobacter and Salmonella infection consist of: weak feeding, irritability, temperature modifications, jaundice, grunting breaths, irregular overall body movements, lethargy, rash or blood in the urine or stool.
- If your toddler is dealing with indications related to Cronobacter or Salmonella infection, contact your child’s health and fitness care supplier to report his or her signs and symptoms and obtain quick care.
When and exactly where ended up the ailments?
Illnesses occurred in Minnesota, Ohio, and Texas amongst September 16, 2021 and January 4, 2022.
I’m having a really hard time getting formula. What is the Fda doing to assist?
We are informed the recall has created new issues about the availability of sure types of infant formula, specifically offered the overall strains on supply chains experienced all through the COVID-19 pandemic.
The Food and drug administration is doing the job with Abbott Nutrition to improved evaluate the impacts of the remember and have an understanding of the production capability at other Abbott facilities that deliver some of the impacted brands. We are also functioning with Abbott on secure resumption of generation at the Sturgis, Michigan facility. As Abbott Nutrition was initiating its recall, the Fda intensified outreach to other infant method producers to inquire about their potential and potential impacts. We will proceed discussion with Abbott Diet and other infant formula brands and contemplate all equipment offered to help the offer of infant method products.
Are home made formulation an alternative?
No. The Fda advises moms and dads and caregivers not to make or feed homemade formula to infants. Homemade infant components recipes have not been evaluated by the Fda and could deficiency vitamins crucial to an infant’s expansion.
What else really should I know?
Parents and caregivers also need to hardly ever dilute infant system. Consumers also need to avoid obtaining components on-line that arrives from outside the house the U.S., as it has the possible to be counterfeit.
If your common components is not accessible, call your child’s well being care supplier for tips on switching feeding procedures.
If you get infant components by way of WIC, do not throw the components out. As a substitute, you must choose it to the shop for a refund and exchange or phone the corporation at 1-800-986-8540 to enable you. WIC recipients must be ready to receive a various brand of equivalent method. Simply call your nearby WIC clinic for more direction.